Neuroscience
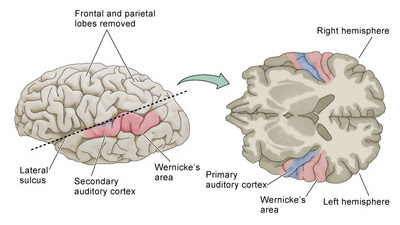
Two new studies demonstrate differences between the brains of people with schizophrenia and those without. In the first, anatomical abnormalities were noted in neurons located in the primary auditory cortex (Sweet et al., 2007). This is an important finding, because one characteristic feature of schizophrenia is auditory hallucinations (Frith, 1996), or hearing voices that are not actually present. This is not the first time such a neuronal abnormality has been observed by this lab (Sweet et al., 2003, 2004), and the current study characterized the density of axon terminals in Brodmann areas 41 and 42. They did this by examining post-mortem tissue from 15 schizophrenia brains and 15 control brains, which were processed immunohistochemically with an agent (synaptophysin) to visualize axon terminals in different cortical layers.
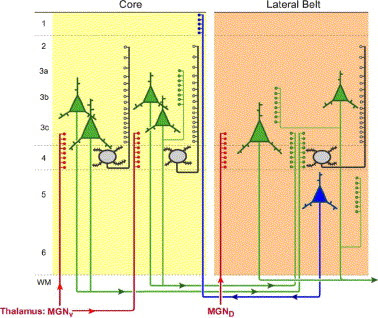
Figure 1 (Sweet et al., 2007). Schematic diagram of feedforward and feedback pathways in the auditory core (area 41) and lateral belt cortices of primate. Auditory sensory processing is primarily initiated via projections (red) from the ventral subdivision of the medial geniculate nucleus of the thalamus to layers 4 and 3c of the core. Excitation spreads to supragranular layers via ascending projections from spiny stellate cells (black) and via ascending collaterals from layer 3 pyramidal cells (thin green). Feedforward projections (thick green) arise predominantly from layer 3 pyramidal cells in core and project to deep layer 3 and layer 4 of the lateral belt. Feedback projections (blue) arise predominantly from layer 5 in lateral belt and terminate in layer 1 of core.
The importance of comparing the pattern of synaptophysin staining in different cortical layers is to determine whether feedforward pathways (which convey information from auditory relay centers in the medial geniculate forward to auditory cortex) or feedback pathways (which send information from higher-order areas back to primary auditory cortex) are more affected. This difference may have some significance for different theories of the origins of auditory hallucinations in schizophrenia [although there won't be an easy answer to that one...].
In short, the study demonstrated that axon terminal densities are reduced in feedforward but not feedback auditory pathways.
In the second study, Perkins and colleagues (2007) identified a molecular mechanism that may contribute to the development of schizophrenia.
References
Frith C. (1996). The role of the prefrontal cortex in self-consciousness: the case of auditory hallucinations. Philos Trans R Soc Lond B Biol Sci. 351:1505-12.
Javitt DC, Shelley AM, Ritter W (2000). Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol 111:1733–1737.
Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. (2002). Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 51:1008-11.
Lewis DA, Hashimoto T, Volk DW. (2005). Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 6:312-24.
Rabinowicz EF, Silipo G, Goldman R, Javitt DC (2000). Auditory sensory dysfunction in schizophrenia: Imprecision or distractibility? Arch Gen Psychiatry 57:1149–1155.
Rusch N, Spoletini I, Wilke M, Bria P, Di Paola M, Di Iulio F, Martinotti G, Caltagirone C, Spalletta G. (2007). Prefrontal-thalamic-cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res. Mar 23; [Epub ahead of print].
Sweet RA, Bergen SE, Sun Z, Sampson AR, Pierri JN, Lewis DA (2004). Pyramidal cell size reduction in schizophrenia: Evidence for involvement of auditory feedforward circuits. Biol Psychiatry 55:1128–1137.
Sweet RA, Pierri JN, Auh S, Sampson AR, Lewis DA. (2003). Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharmacology 28:599–609.
- Computational Vision
The new study A Feedforward Architecture Accounts for Rapid Categorization, Serre, T., A. Oliva and T. Poggio, PNAS 2007, in press [not online yet] reveals the success of a computational version of vision modeled on the visual cortex processes of immediate...
- No Schizophrenia?
2 NAMES, 1 DISEASE: Does schizophrenia=psychotic bipolar disorder? in Current Psychiatry suggests that schizophrenia is actually severe bipolar I psychosis and not a separate diagnosis. A comprehensive review references questions going back many years,...
- Glutamate Agonist Ly2140023: A New Treatment For Schizophrenia?
High hopes for new schizophrenia drugs Drug trial hailed as first major breakthrough for 50 years. By Alison Abbott . . . The side effects of LY2140023, including insomnia and emotional instability, are slightly different to those of olanzapine although...
- Fda: Saphris (asenapine) Approval, Schizophrenia And Bipolar
From the FDA: FDA Approves Saphris to Treat Schizophrenia and Bipolar Disorder The U.S. Food and Drug Administration has approved Saphris tablets (asenapine) to treat adults with schizophrenia, a chronic, severe and disabling brain disorder, and to treat...
- Neuropsychology Abstract Of The Day: Schizophrenia And Cognition
Palmer BW, Dawes SE, & Heaton RK. (2009). What Do We Know About Neuropsychological Aspects Of Schizophrenia? Neuropsychology Review. Jul 30 [e-pub ahead of print]. Application of a neuropsychological perspective to the study of schizophrenia has established...
Neuroscience
Differences in Auditory Cortex Neurons and Prefrontal microRNA Expression in Schizophrenia

Two new studies demonstrate differences between the brains of people with schizophrenia and those without. In the first, anatomical abnormalities were noted in neurons located in the primary auditory cortex (Sweet et al., 2007). This is an important finding, because one characteristic feature of schizophrenia is auditory hallucinations (Frith, 1996), or hearing voices that are not actually present. This is not the first time such a neuronal abnormality has been observed by this lab (Sweet et al., 2003, 2004), and the current study characterized the density of axon terminals in Brodmann areas 41 and 42. They did this by examining post-mortem tissue from 15 schizophrenia brains and 15 control brains, which were processed immunohistochemically with an agent (synaptophysin) to visualize axon terminals in different cortical layers.

Figure 1 (Sweet et al., 2007). Schematic diagram of feedforward and feedback pathways in the auditory core (area 41) and lateral belt cortices of primate. Auditory sensory processing is primarily initiated via projections (red) from the ventral subdivision of the medial geniculate nucleus of the thalamus to layers 4 and 3c of the core. Excitation spreads to supragranular layers via ascending projections from spiny stellate cells (black) and via ascending collaterals from layer 3 pyramidal cells (thin green). Feedforward projections (thick green) arise predominantly from layer 3 pyramidal cells in core and project to deep layer 3 and layer 4 of the lateral belt. Feedback projections (blue) arise predominantly from layer 5 in lateral belt and terminate in layer 1 of core.
The importance of comparing the pattern of synaptophysin staining in different cortical layers is to determine whether feedforward pathways (which convey information from auditory relay centers in the medial geniculate forward to auditory cortex) or feedback pathways (which send information from higher-order areas back to primary auditory cortex) are more affected. This difference may have some significance for different theories of the origins of auditory hallucinations in schizophrenia [although there won't be an easy answer to that one...].
In short, the study demonstrated that axon terminal densities are reduced in feedforward but not feedback auditory pathways.
Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. (2007). Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry 61:854-64.The authors speculate on the significance of their findings for impairments in auditory processing in schizophrenia [although they do not speculate about the origin of auditory hallucinations...]:
BACKGROUND: Somal volumes of pyramidal cells are reduced within feedforward but not feedback circuits in areas 41 and 42 of the auditory cortex of subjects with schizophrenia. Because neuronal somal volume depends on both the number of axonal terminations onto and furnished by the neuron, we hypothesized that axon terminal densities are reduced in feedforward but not feedback auditory pathways in subjects with schizophrenia. METHODS: We used stereologic methods to quantify the density of a marker of axon terminals, synaptophysin-immunoreactive (SY-IR) puncta, in areas 41 and 42 of 15 subjects with schizophrenia and matched normal comparison subjects. The effect of long-term haloperidol exposure on density of SY-IR puncta was similarly evaluated in nonhuman primates. RESULTS: Synaptophysin-immunoreactive puncta density was 13.6% lower in deep layer 3 of area 41 in the schizophrenia subjects but was not changed in layer 1 of area 41 or in deep layer 3 of area 42. Density of SY-IR puncta did not differ between haloperidol-exposed and control monkeys. CONCLUSIONS: Reduction of SY-IR puncta density is selective for feedforward circuits within primary auditory cortex of subjects with schizophrenia. This deficit may contribute to impairments in auditory sensory processing in this disorder.
If our findings do reflect a lower number of excitatory feedforward thalamocortical and intrinsic axon terminals, then these abnormalities might underlie the deficits in auditory processing observed in subjects with schizophrenia. Individuals with schizophrenia demonstrate impaired pure tone discrimination (Rabinowicz et al. 2000). Though the precise intracortical mechanisms of this impairment are not known, this deficit is correlated with reductions in mismatch negativity (MMN), an evoked response potential arising in response to auditory stimuli that deviate in one characteristic (e.g., pitch) from a repetitive stimulus (Javitt et al. 2000).But can't these neuroanatomical differences be produced by antipsychotic drugs? Thirteen of the brains were obtained from individuals treated with medications, and only two without. So "merely a by-product of drug treatment" is a valid objection, but the investigators considered this in a control experiment. Four monkeys were treated with haloperidol (a typical antipsychotic) for 9-12 months, then their brains were examined and compared to those of control monkeys. No significant differences were observed in the primary auditory cortices of the two groups. In addition, the brains from the individuals off medications at the time of their deaths did not differ from those obtained from medicated individuals (although the authors acknowledged the lack of statistical power in this comparison).
In the second study, Perkins and colleagues (2007) identified a molecular mechanism that may contribute to the development of schizophrenia.
Brain tissue reveals possible genetic trigger for schizophreniaThe reported differences were in prefrontal cortex, which has been repeatedly demonstrated to be altered in schizophrenia (e.g., Lewis et al., 2005; Rusch et al., 2007) [as have interactions between prefrontal and auditory areas (Lawrie et al., 2002]. Once again, the investigators did appropriate control studies (in haloperidol-treated and untreated rats this time) to demonstrate that the alterations were not due to medication effects in schizophrenia.
. . .
In studying the postmortem brain tissue of adults who had been diagnosed with schizophrenia, the researchers found that levels of certain gene-regulating molecules called microRNAs were lower among schizophrenia patients than in persons who were free of psychiatric illness.
"In many genetic diseases, such as Huntington's disease or cystic fibrosis, the basis is a gene mutation that leads to a malformed protein. But with other complex genetic disorders – such as schizophrenia, many cancers, and diabetes – we find not mutated proteins, but correctly formed proteins in incorrect amounts," said study lead author and UNC professor of psychiatry Dr. Diana Perkins.
Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Parker JS, Jin J, Hammond SM. (2007). microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 8(2):R27.Some people believe that there are no documented differences between the brains of people with and those without psychiatric disorders. That mental illnesses do not have biological causes. Certainly, one's environment, social circumstances, upbringing, stress levels, etc. do play a role in the expression of various mental illnesses, but so do genetics, brain structure, and brain function. The present studies provide yet another example of the latter.
BACKGROUND: microRNAs (miRNAs) are small, noncoding RNA molecules that are now thought to regulate the expression of many mRNAs. They have been implicated in the etiology of a variety of complex diseases, including Tourette's syndrome, Fragile x syndrome, and several types of cancer. RESULTS: We hypothesized that schizophrenia might be associated with altered miRNA profiles. To investigate this possibility we compared the expression of 264 human miRNAs from postmortem prefrontal cortex tissue of individuals with schizophrenia (n = 13) or schizoaffective disorder (n = 2) to tissue of 21 psychiatrically unaffected individuals using a custom miRNA microarray. Allowing a 5% false discovery rate, we found that 16 miRNAs were differentially expressed in prefrontal cortex of patient subjects, with 15 expressed at lower levels (fold change 0.63 to 0.89) and 1 at a higher level (fold change 1.77) than in the psychiatrically unaffected comparison subjects. The expression levels of 12 selected miRNAs were also determined by quantitative RT-PCR in our lab. For the eight miRNAs distinguished by being expressed at lower microarray levels in schizophrenia samples versus comparison samples, seven were also expressed at lower levels with quantitative RT-PCR. CONCLUSION: This study is the first to find altered miRNA profiles in postmortem prefrontal cortex from schizophrenia patients.
References
Frith C. (1996). The role of the prefrontal cortex in self-consciousness: the case of auditory hallucinations. Philos Trans R Soc Lond B Biol Sci. 351:1505-12.
Javitt DC, Shelley AM, Ritter W (2000). Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol 111:1733–1737.
Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. (2002). Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 51:1008-11.
Lewis DA, Hashimoto T, Volk DW. (2005). Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 6:312-24.
Rabinowicz EF, Silipo G, Goldman R, Javitt DC (2000). Auditory sensory dysfunction in schizophrenia: Imprecision or distractibility? Arch Gen Psychiatry 57:1149–1155.
Rusch N, Spoletini I, Wilke M, Bria P, Di Paola M, Di Iulio F, Martinotti G, Caltagirone C, Spalletta G. (2007). Prefrontal-thalamic-cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res. Mar 23; [Epub ahead of print].
Sweet RA, Bergen SE, Sun Z, Sampson AR, Pierri JN, Lewis DA (2004). Pyramidal cell size reduction in schizophrenia: Evidence for involvement of auditory feedforward circuits. Biol Psychiatry 55:1128–1137.
Sweet RA, Pierri JN, Auh S, Sampson AR, Lewis DA. (2003). Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharmacology 28:599–609.
- Computational Vision
The new study A Feedforward Architecture Accounts for Rapid Categorization, Serre, T., A. Oliva and T. Poggio, PNAS 2007, in press [not online yet] reveals the success of a computational version of vision modeled on the visual cortex processes of immediate...
- No Schizophrenia?
2 NAMES, 1 DISEASE: Does schizophrenia=psychotic bipolar disorder? in Current Psychiatry suggests that schizophrenia is actually severe bipolar I psychosis and not a separate diagnosis. A comprehensive review references questions going back many years,...
- Glutamate Agonist Ly2140023: A New Treatment For Schizophrenia?
High hopes for new schizophrenia drugs Drug trial hailed as first major breakthrough for 50 years. By Alison Abbott . . . The side effects of LY2140023, including insomnia and emotional instability, are slightly different to those of olanzapine although...
- Fda: Saphris (asenapine) Approval, Schizophrenia And Bipolar
From the FDA: FDA Approves Saphris to Treat Schizophrenia and Bipolar Disorder The U.S. Food and Drug Administration has approved Saphris tablets (asenapine) to treat adults with schizophrenia, a chronic, severe and disabling brain disorder, and to treat...
- Neuropsychology Abstract Of The Day: Schizophrenia And Cognition
Palmer BW, Dawes SE, & Heaton RK. (2009). What Do We Know About Neuropsychological Aspects Of Schizophrenia? Neuropsychology Review. Jul 30 [e-pub ahead of print]. Application of a neuropsychological perspective to the study of schizophrenia has established...
