Neuroscience
A year ago, Ketamine for Depression: Yay or Neigh? covered acute administration of the club drug (and dissociative anesthetic) ketamine for rapid (albeit transient) relief of major depression. That post was part of a blog focus on hallucinogenic drugs in medicine and mental health, organized by Nature editor Noah Gray following publication of a review article on The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. At the time, I wrote:
In terms of medical ethics, it's easier for me to take a different angle and address the unusual case of a grievously and chronically depressed patient (Messer & Haller, 2010). An anonymous reader alerted me to this paper, which isn't indexed in PubMed. The case history is as follows:
On the other hand Dr. Messer's clinical trial, Ketamine Frequency Treatment for Major Depressive Disorder, was withdrawn prior to enrollment because pilot study determined the trial would not be feasible. The planned regimen was 6 injections every other day for 12 days. But the actual treatment given to the 46 yr old woman was much more extensive: 22 doses over 4 months, followed by 21 doses over 1 yr (approximately):
What were the cognitive effects? We don't really know, because there was no formal testing:
References
aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. (2010). Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139-45.
Irwin SA, Iglewicz A. (2010). Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 13:903-8.
Javitt DC. (2010). Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci. 47:4-16.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329(5994):959-64.
Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, Liu T, Chen X, Fletcher PC, Hao W. (2010). Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain 133:2115-22.
Messer M, Haller IV (2010). Maintenance Ketamine Treatment Produces Long-term Recovery from Depression. Primary Psychiatry, 17, 48-50.
Tsai GE. (2007). Searching for rational anti N-methyl-D-aspartate treatment for depression. Arch Gen Psychiatry 64:1099-100; author reply 1100-1.
Vollenweider F, Kometer M. (2010). The neurobiology of psychedelic drugs: implications for the treatment of mood disorders Nature Reviews Neuroscience, 11 (9), 642-651.
Yang C, Zhou ZQ, Yang JJ. (2011). Be prudent of ketamine in treating resistant depression in patients with cancer. J Palliat Med. 14:537.
- Light Relief For Long-term Depression
There’s emerging evidence that sitting near a bright light every morning could help people with depression (see recent Cochrane review). More dubious is the suggestion that ‘negative ion generators’ – gadgets that purportedly increase the concentration...
- On The Long Way Down: The Neurophenomenology Of Ketamine
Is ketamine a destructive club drug that damages the brain and bladder? With psychosis-like effects widely used as a model of schizophrenia? Or is ketamine an exciting new antidepressant, the “most important discovery in half a century”? For years,...
- Warning About Ketamine In The American Journal Of Psychiatry
The dissociative anesthetic and ravey club drug ketamine has been hailed as a possible “miracle” cure for depression. In contrast to the delayed action of standard antidepressants such as SSRIs, the uplifting effects of Special K are noticeable within...
- Update On Ketamine In Palliative Care Settings
Many recent headlines have heralded a new use for the old veterinary anesthetic ketamine, which can provide rapid-onset (albeit short-lived) relief for some patients with treatment-resistant depression (aan het Rot et al., 2012). This finding has been...
- While I Was Away...
While I was (mostly) away from the internet, I missed over a week of online excitement. These items are old news by now, but in case you've forgotten... (1) NPR Morning Edition had a piece on acutely administered ketamine for the treatment of depression:...
Neuroscience
Chronic Ketamine for Depression: An Unethical Case Study?

A year ago, Ketamine for Depression: Yay or Neigh? covered acute administration of the club drug (and dissociative anesthetic) ketamine for rapid (albeit transient) relief of major depression. That post was part of a blog focus on hallucinogenic drugs in medicine and mental health, organized by Nature editor Noah Gray following publication of a review article on The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. At the time, I wrote:
Although the immediate onset of symptom amelioration gives ketamine a substantial advantage over traditional antidepressants (which take 4-6 weeks to work), there are definite limitations (Tsai, 2007). Drawbacks include the possibility of ketamine-induced psychosis (Javitt, 2010), limited duration of effectiveness (aan het Rot et al., 2010), potential long-term deleterious effects such as white matter abnormalities (Liao et al., 2010), and an inability to truly blind the ketamine condition due to obvious dissociative effects in many participants.For the past few weeks, I've been wanting to do a follow-up post that looks at the ups and downs of the mTOR (mammalian target of rapamycin) protein kinase pathway, which is rapidly activated by ketamine. Although activation of mTOR leads to the beneficial effect of increased synaptogenesis in the medial prefrontal cortex (Li et al., 2010), it can also cause accelerated tumor growth, as recently noted by Yang et al., 2011 ("Be prudent of ketamine in treating resistant depression in patients with cancer"). However, I've been unable to complete this planned post, specifically because the topic of ketamine use in palliative care settings is something I wrote about last year, while watching my father die of cancer.
At present, what are the most promising uses for ketamine as a fast-acting antidepressant? Given the disadvantages discussed above, short-term use for immediate relief of life-threatening or end-of-life depressive symptoms seem to be the best indications.
More recently, an open label study in two hospice patients, each with a prognosis of only weeks or months to live, showed beneficial effects of ketamine in the treatment of anxiety and depression (Irwin & Iglewicz, 2010). A single oral dose produced rapid improvement of symptoms and improved end of life quality.To be blunt, the possibility of accelerated tumor growth is not an issue in terminal patients.
In terms of medical ethics, it's easier for me to take a different angle and address the unusual case of a grievously and chronically depressed patient (Messer & Haller, 2010). An anonymous reader alerted me to this paper, which isn't indexed in PubMed. The case history is as follows:
In January 2008, a 46-year old female with MDD was hospitalized for a course of electroconvulsive therapy (ECT). Successive interventions over 15 years had included trials of 24 psychotropic medications and 273 ECT treatments, 251 of which were bilateral [which can produce significant amnesia]. No intervention had produced remission but only a short-lived response to treatment...ECT during this admission was administered with ketamine as the anesthetic at 2 mg/kg given over 60 seconds. Surgical anesthesia occurred ~30 seconds after the end of intravenous injection and lasted ~10 minutes. There was no significant change in depression symptoms with the ketamine used as an anesthetic during the ECT treatment. Alternative treatments were reviewed for potential use. In addition to no significant recovery from her depression, the long-term use of ECT caused problems with memory loss and focused attention. She was unable to remember much of her history over the previous 15 years. Re-learning the information became futile since each course of ECT would eliminate what had been gained.I'm not going to weigh in here on ECT, beyond saying that it can be beneficial in some intractable patients [with fewer amnestic effects if unilateral]. But here we have an individual with profound ECT-induced amnesia who, although giving informed consent, was then treated with a highly unorthodox regimen of repeated ketamine infusions. The majority of registered clinical trials administer a single dose of ketamine, with one trial administering 5 additional ketamine infusions over a 2-week period. Relapse typically occurs within a week after a single dose.
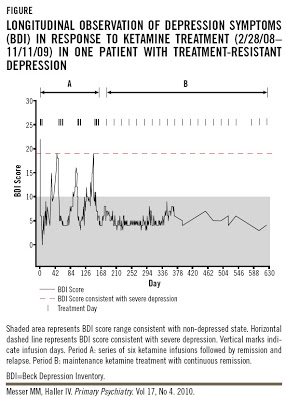
On the other hand Dr. Messer's clinical trial, Ketamine Frequency Treatment for Major Depressive Disorder, was withdrawn prior to enrollment because pilot study determined the trial would not be feasible. The planned regimen was 6 injections every other day for 12 days. But the actual treatment given to the 46 yr old woman was much more extensive: 22 doses over 4 months, followed by 21 doses over 1 yr (approximately):
The first ketamine treatment led to a dramatic remission of depressive symptoms: the Beck Depression Inventory (BDI) score decreased from 22 to 6 (Figure). Three additional infusions administered every other day over 5 days produced remission lasting 17 days after the last infusion in this series. Three series of six ketamine infusions given every other day except weekends were repeated over the next 16 weeks (Figure). Each infusion sequence produced remission lasting 16, 28, and 16 days, respectively, followed by a relapse. After three remission/relapse cycles and before relapse could occur after the fourth infusion series, a maintenance ketamine regimen was established on August 27, 2008 using 0.5 mg/kg IBW at a 3-week inter-dose interval. The authors’ estimation for the maintenance dosing interval was based on the time frame between remission and relapse for this patient. Relapse to depression was prevented by treating prior to the onset of a relapse.First, I was struck by the starting BDI score of 22, which falls within the low end of moderate depression, with scores of 29-63 indicating severe depression. I don't want to question Dr. Messer's clinical diagnosis of the patient, but I would guess that a typical BDI II score of 22 might not call for drastic measures. But perhaps the original BDI was used, in which case 19-29 indicates moderate-severe depression (which is still not severe). Second, the number of infusions went well beyond what has been established as safe, particularly in the context of treatment-resistant depression.
- click on image for a larger view -

What were the cognitive effects? We don't really know, because there was no formal testing:
As shown in the Figure, with maintenance infusions the patient has been in remission for >15 months. No concurrent pharmacotherapeutic agents have been administered or required during this time period, no adverse events have emerged, and there has been no cognitive impairment as is typical with ECT, polypharmacy, or from MDD itself.What we do know is that ketamine is cost-effective relative to ECT:
The cost and personnel needed for a ketamine treatment are far less than that of ECT since no charges associated with anesthesia or operating room use are needed. The data from our institution suggest that the charges associated with one ketamine treatment are ~33% of the charges for one ECT.But it's caution, and not cost-effectiveness, that should be of the utmost importance in vulnerable, chronically depressed patients who are treatment-resistant.
References
aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. (2010). Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139-45.
Irwin SA, Iglewicz A. (2010). Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 13:903-8.
Javitt DC. (2010). Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci. 47:4-16.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329(5994):959-64.
Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, Liu T, Chen X, Fletcher PC, Hao W. (2010). Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain 133:2115-22.
Messer M, Haller IV (2010). Maintenance Ketamine Treatment Produces Long-term Recovery from Depression. Primary Psychiatry, 17, 48-50.
Tsai GE. (2007). Searching for rational anti N-methyl-D-aspartate treatment for depression. Arch Gen Psychiatry 64:1099-100; author reply 1100-1.
Vollenweider F, Kometer M. (2010). The neurobiology of psychedelic drugs: implications for the treatment of mood disorders Nature Reviews Neuroscience, 11 (9), 642-651.
Yang C, Zhou ZQ, Yang JJ. (2011). Be prudent of ketamine in treating resistant depression in patients with cancer. J Palliat Med. 14:537.
Dedication:
For my father, who has been deeply missed since September 6, 2010.
- Light Relief For Long-term Depression
There’s emerging evidence that sitting near a bright light every morning could help people with depression (see recent Cochrane review). More dubious is the suggestion that ‘negative ion generators’ – gadgets that purportedly increase the concentration...
- On The Long Way Down: The Neurophenomenology Of Ketamine
Is ketamine a destructive club drug that damages the brain and bladder? With psychosis-like effects widely used as a model of schizophrenia? Or is ketamine an exciting new antidepressant, the “most important discovery in half a century”? For years,...
- Warning About Ketamine In The American Journal Of Psychiatry
The dissociative anesthetic and ravey club drug ketamine has been hailed as a possible “miracle” cure for depression. In contrast to the delayed action of standard antidepressants such as SSRIs, the uplifting effects of Special K are noticeable within...
- Update On Ketamine In Palliative Care Settings
Many recent headlines have heralded a new use for the old veterinary anesthetic ketamine, which can provide rapid-onset (albeit short-lived) relief for some patients with treatment-resistant depression (aan het Rot et al., 2012). This finding has been...
- While I Was Away...
While I was (mostly) away from the internet, I missed over a week of online excitement. These items are old news by now, but in case you've forgotten... (1) NPR Morning Edition had a piece on acutely administered ketamine for the treatment of depression:...
